What Does the Solvent Do in a Solution Apex
What effect does adding a solute to a solvent have on the freezing point of the solvent apex. What effect does adding a solute to a solvent have on the boiling point of the solvent.

Learn About Solutions Science For Kids Chemistry Classroom Science For Kids Teaching Science
However it can be a gas solid or supercritical fluid.

. Molality is used to measure concentration of solute molecule present in a solution. Such that when anything is dissolved in a solvent say water the solution will boil at a higher temperature than pure solvent water would. The solubility would then equal the concentration of either the Ag or Cl ions.
Higher boiling point of the solution relative to that of the pure solvent. Solute is the substance that gets dissolve in solventLikewise solvent is the substance that dissolve the soluteThe mixture of solute and solvent gives solution. For egthe mixture of water and soilthe water act as solvant as it dissolve soil and soil is solute as it gets dissolve in solvent.
Effects of Solute on Solvent 2 points a. Once you dissolve the electrolytes albumin glucose and other solutes into the water solvent of the human body the result is a solution. Solubility is the amount of reagent that will be consumed to saturate the solution or reach the equilibrium of the dissociation reaction.
A solute is the material present in the smaller amount in the solution. It depends only on the number of particles in the solution. A solution with a molality of 3 molkg is often described as 3 molal or 3 m.
What increases the boiling point of water. The solvent is water because a solvent is a liquid that does the dissolving in a solution. I believe the answer is C the temperature at which the solution boils is raised.
For this reaction each mole of AgCl that dissolves produces 1 mole of both Ag and Cl -. A solvent is the component of a solution that is present in the greatest amount. What effect does adding a solute to a solvent have on the freezing point of the solvent.
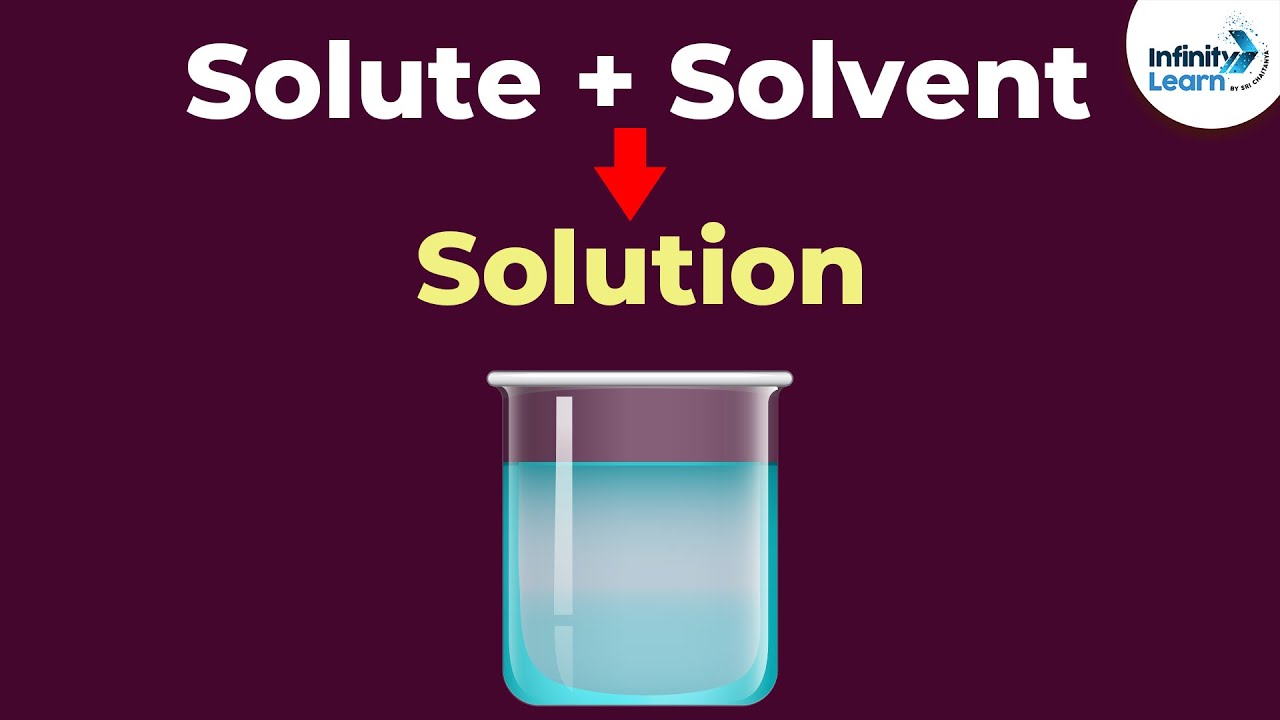
The Solvent dissolves the Solute substance. Solvent substance ordinarily a liquid in which other materials dissolve to form a solution. Solvent and Solute make a Solution Solvent Solute Solution.
Molality is a property of a solution and is defined as the number of moles of solute per kilogram of solvent. What equation describes the effect. Solvents may be predominantly acidic predominantly basic amphoteric both or.
The solute raises the boiling point by an amount that depends on the number of particles it contributes go the solution. The solute is the substance that is being dissolved while the solvent is the dissolving medium. Polar solvents eg water favour formation of ions.
For exam if 6 moles of a solute is present in 1 kg of solvent then the concentration of that solute molecule will be 6 molal. A solution is a homogeneous mixture consisting of a solute dissolved into a solvent. Every commonly used IV fluid is a solution.
The presence of a solute lowers the freezing point of any solvent. It is the substance in which the solute is dissolved. A solvent is the material present in the larger amount in the solution.
Boiling point elevation is a colligative property. When one substance dissolves into another a solution is formed. Colligative properties describe this reaction.
Navy and countless OEMs RYDLYME descaling chemical is. RYDLYME Biodegradable Industrial Descaler. Recommended by the US.
When a solute is added to a solvent the boiling point will be higher than the boiling point of the pure solvent. Solute particles are distributed throughout the solution. Lower freezing point of the solution relative to that of the pure solvent.
1 point Adding a solvent to the solute can decrease the freezing point like adding salt to ice on the roads. The amount of solvent required to dissolve a solute depends on temperature and the presence of other substances in a sample. Nonpolar ones eg hydrocarbons do not.
A property of a solution that depends on the number not the identity of the solute particles. You havent told me what exactly is dissolved in your extract aside from the dichloromethane solvent itself so Ill have to be a bit general. Typically the 1-cm layer of anhydrous sodium sulfate is there to help hold polar molecules and seperate them from less polar ones as you perform the extraction into the round-bottom flask.
What effect does adding a solute to a solvent have on the boiling point of the solvent apex. Usually a solvent is a liquid. This effect is called freezing-point depression.
RYDLYME Biodegradable Descaler is used to safely and efficiently dissolve tough mineral deposits such as water scale limescale calcium and rust from boilers chillers cooling towers condensers heat exchangers struvite and more. Normal Saline 3 Saline and 045 Saline are all good examples. A process used to separate a mixture whereby the mixture is dissolved in a gas or liquid and the components are separated by differences in adsorption to a solid surface or viscous liquid.
Molality represent the presence of total number of solute molecules in a kilgram of solvent. Solutions can be formed with many different types and forms of solutes and solvents. If the solution is dilute in terms of the concentration of solid you added then the heat capacity will be dominated by that of the solvent or the heat capacity of the solution will be little altered by addition of a minute amount of solute relative to the value for the pure solvent.
The SI unit for molality is molkg. And you may have already guessed this but the plasma part of your blood is also a solution. Adding a solute to.

Solute Solvent Solution For Kids Kindergarten Toddlers Preschoolers Teaching Chemistry Nursing School Studying Toddler Preschool

Apex 4 3 Mixtures Of Matter Science Quizizz
Explain On A Particle Basis How The Addition Of A Solute Lisbdnet Com

How To Calibrate A Ph Meter Without Solution Atlas Scientific

2 Oz Plastic Flux Solvent Dispenser Includes Dispensing Needle Dispenses 1 Drop Of Liquid At A Time Perfect For Jewelry Glass Supplies Flux Squeeze Bottles

Pin By Rebecca Paschal On Essential Oils Lavender Oil Tea Tree Oil Rosemary Oil

Pin By Brittany Landise On Class 1o Notes Science Notes Science Class

Member S Mark Commercial Carpet Cleaner Formerly Proforce Commercial 1 Gal Bottle Commercial Carpet Cleaners Commercial Carpet Carpet Cleaner

Switchable Solvents Jessop Lab

Preparation Of Lyophobic Sols General Knowledge Exam Preparation

Mixtures And Solutions Chemistry For Kids Solute Solvent Solution School Science Experiments Matter Science Chemistry Education

Desinfectante De Manos Liquido A Granel Raleigh Durham Fayetteville Cary Apex Garner Clayton Knightdale Industrysupplyinc In 2022 Hand Sanitizer Packaging Supplies Family Christmas Crafts

Solutions Solvent And Solute Teaching Chemistry Chemistry Classroom Interactive Science Notebook

ब स टर ड ज क त र पर क व व क स क फ ज 3 ट र यल क स फ र श वयस क क द ज एग व क स न In 2022 Hand Soap Bottle Soap Bottle Soap

Insparation Holiday Sugar Cookie Aromatherapy 9 Oz Bottle Holiday Sugar Cookies Aromatherapy Scents Holiday Blend

Switchable Solvents Jessop Lab

Apex 4 3 Mixtures Of Matter Science Quizizz

Solutions Solutes And Solvents New Chemistry Science Poster Science Chemistry Chemistry Classroom Science Lessons

Comments
Post a Comment